Types of Redox Reactions
Types of Redox Reactions: Overview
This topic covers concepts, such as, Types of Redox Reactions, Combination Reactions, Disproportionation Reactions & Comproportionation Reaction etc.
Important Questions on Types of Redox Reactions
Which of the following options are correct for the reaction?
A. Redox reaction
B. Displacement reaction
C. Decomposition reaction
D. Combination reaction
Choose the correct answer from the options given below:
| Reaction | Type of redox reaction | ||
| Intermolecular | |||
| Intramolecular | |||
| Disproportion | |||
| Comproportion |
Soluble salt like sodium sulphite can be prepared in the laboratory by the process of:
Which of the following is not a redox reaction?
Which one of the following is an example of disproportionation reaction?
Which of the following represents disproportionation of potassium chlorate?
Which species among the following doesn't show disproportionation reactions?
Which of the following reactions are disproportionation reactions?
Non metal which do not give disproportionation reaction with is -
The reaction, , is an example of :
in neutral aqueous medium disproportionates to :
In which of the following reactions, hydrogen peroxide acts an oxidizing agent?
Which of the following is not an example of disproportionation :
When gas reacts with hot and concentrated sodium hydroxide solution, the oxidation number of chlorine changes from:
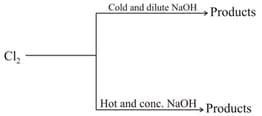
The product which is not common in (i.e. present in one but not in the other) both the reactions is :
When white phosphorus reacts with , phosphine gas is released along with a compound . Compound X on reaction with form an acid . What is the basicity of ?
Which of the following is a disproportionation reaction?
On heating gives -
Which of the following species undergoes non redox thermal decomposition reaction on heating ?
Which reaction does not represent auto redox or disproportionation ?
